Bone Regeneration Technology Used in First Patient Surgery
First clinical use of citrate-based biomaterial marks milestone in regenerative engineering
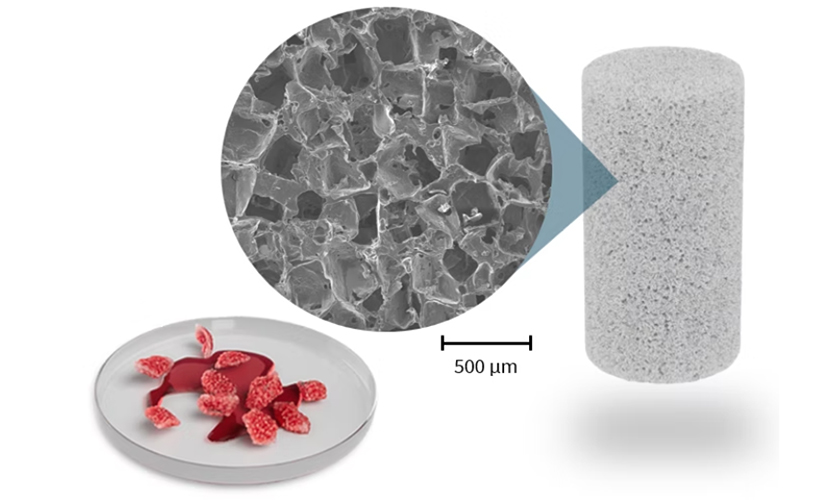
A bone regeneration device using bioactive materials pioneered by Querrey Simpson Institute for Regenerative Engineering at Northwestern University (QSI RENU) director Guillermo Ameer was recently used in its first surgical case, Acuitive Technologies Inc. announced.

Called Citregraft, the technology is composed of the citrate-based polymers developed in the Ameer laboratory as well as bioactive glass. It is a highly porous, synthetic bone graft substitute that can be morselized to fit irregular defects. After placement in a bony void, Citregraft binds local growth factors and steadily releases citrate to support bone regeneration before resorbing and being replaced by the patient’s natural bone.
In its first surgical use, the sponge-like material repaired bone tissue that was harvested as part of an ACL reconstruction on the knee. The device received FDA clearance last November and is one of several products in Acuitive Technologies’ CITREGEN product line.
“Twenty years after the first report of citrate-based polymers we now see another major milestone: the use of our biomaterial technology to develop a citrate-based bioactive scaffold to regenerate bone tissue in patients,” said Ameer, the Daniel Hale Williams Professor of Biomedical Engineering at Northwestern Engineering and a professor of surgery in Northwestern’s Feinberg School of Medicine. “This builds on the success and expands the impact our biomaterial technology and collaboration with Acuitive Technologies has made on medical devices that regenerate musculoskeletal tissues.
“I am very proud of our research team members, past and present, who have contributed to the development and applications of citrate-based biomaterials. I am humbled to see that the impact of our publications goes beyond academic research, being validated by industry, and now improving the lives of patients.”
Editor’s note: Intellectual property associated with the Citregraft is subject to an exclusive license between Northwestern and Vesseltek Biomedical. Pursuant to the terms of that license, Northwestern has financial interest in Vesseltek Biomedical. Vesseltek Biomedical sublicenses Northwestern intellectual property associated with Citregraft to Acuitive Technologies. Ameer has financial interest in Vesseltek Biomedical and serves on the scientific advisory board of Acuitive Technologies.