Platform Leverages AI to Manipulate Cells at Scale
Localized electroporation device could support pivotal cellular engineering processes
Manipulating cells to produce cell-based therapeutics involves the careful introduction of biomolecular cargoes into cells using precise dosage. However, efficiently delivering molecules into live cells while preserving their viability and function remains a challenge.
One technique to overcome cell degradation is localized electroporation, which consists of the application of electrical pulses at localized regions of the cell membrane and results in the formation of resealable pores. Researchers can then nondestructively deliver or extract molecules into or out of living cells.
Now, a research team led by Northwestern Engineering’s Horacio Espinosa has introduced a scaled version of its localized electroporation device (LEPD) platform that can process millions of cells with multiplexed delivery conditions simultaneously. Enhanced with artificial intelligence (AI), the system could significantly improve and accelerate cellular engineering processes that are pivotal to applications like disease diagnostics, cell-based therapies, drug development, gene editing, and systems biology.

“Our LEPD system offers a compact and flexible cellular delivery and analysis platform that can be tuned toward diverse biological applications that require controlled, high-throughput cell manipulation and analysis,” said Espinosa, James N. and Nancy J. Farley Professor in Manufacturing and Entrepreneurship and professor of mechanical engineering at the McCormick School of Engineering. “This design significantly reduces the time required to optimize the experimental conditions needed to achieve efficient molecular delivery into different cell types.”
The team’s findings were presented in the paper “Multiplexed High Throughput Localized Electroporation Workflow with Deep-Learning based Analysis for Cell Engineering,” published July 22 in the journal Science Advances.
The redesigned LEPD features a modular multi-well format and an AI-assisted automated image segmentation and analysis workflow. To test the platform, the researchers demonstrated optimized plasmid delivery into a range of immortalized cell lines — engineered cells that grow indefinitely under in vitro — as well as into stem cells. Across the range of cell types studied, the system was shown to surpass or be comparable to commercially available delivery systems in delivery efficiency, viability, precision, and control over dosage.
“We achieved throughputs that exceed previous demonstrations of localized electroporation platforms, which is essential for applications that require rapid processing of large numbers of cells, such as gene-editing and T-cell immunotherapy,” Espinosa said.
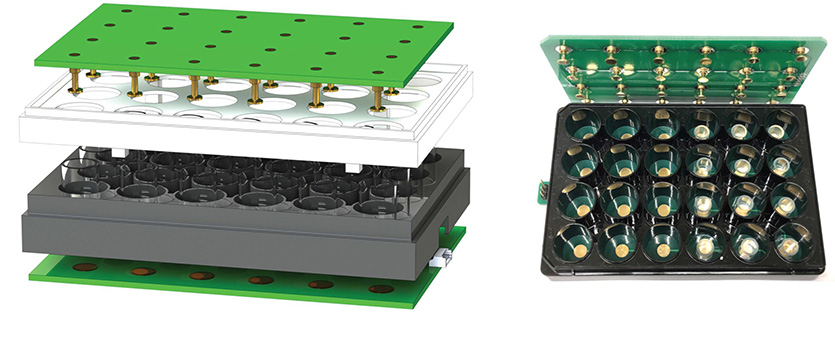
The researchers also demonstrated the multi-well LEPD’s versatility by optimizing delivery of functional biomolecular cargo into a variety of cell types, including clinically relevant human immune pluripotent stem cells (hiPSCs). The system’s deep learning framework offered enhanced imaging analysis, revealing changes in morphological features and protein expression in the genetically perturbed cells with single cell resolution.
“Our work shows the potential of the LEPD system to be used in studies related to stem cell engineering where it’s important to monitor protein expression levels, sub-cellular localization, and morphological changes,” said Cesar Patino, a coauthor on the study and a PhD student in Espinosa’s research group.
Coauthor Nibir Pathak, also a PhD student in Espinosa’s group, added, “Leveraging AI to analyze image data significantly improves both the accuracy and turnaround time of the process as it enables quick identification and quantification of even subtle differences in characteristics of cell physiology and the signal between cells exposed to different experimental conditions.”
Espinosa said his team next plans to explore using the high-throughput LEPD platform to simultaneously introduce multiple genetic perturbations in cells for functional applications in medicine.
“Understanding the genetic factors that regulate and affect stem cell pluripotency and self-renewal has many implications in medical science, whether its regenerative medicine, deciphering genetic disorders like hypertension or diabetes, or efficiently engineering stem cells for therapeutic purposes,” Espinosa said.
This work was supported by funding from a National Institutes of Health (NIH) R21 grant, number 804 1R21GM132709-01, and an NIH R01 grant, number R01HL152314.