Reshaping the Virus
Tullman-Ercek develops a smaller virus for drug delivery
Viruses are masters of delivery. When we become infected, viruses integrate with our cells in order to insert their own genetic material inside us. So as researchers look for novel ways for more precise drug delivery, using viruses seems like an obvious choice.
“There is a lot of work to develop viral vectors into drug delivery agents,” said Northwestern Engineering’s Danielle Tullman-Ercek. “What we don’t know is how the virus’s properties, like shape and size, affect this delivery process.”
Tullman-Ercek, associate professor of chemical and biological engineering in Northwestern’s McCormick School of Engineering, has created a new method to help researchers discover how much the virus’s size matters. By mutating a single amino acid in a bacterial virus, Tullman-Ercek’s team drastically changed the size of its protein coating, or capsid.
 “To date, there has not really been a way to compare what happens with different sizes and shapes of the same virus,” Tullman-Ercek said. “Size matters for a lot of drug delivery purposes because it changes which cells are accessible and how much information can be housed inside the virus.”
“To date, there has not really been a way to compare what happens with different sizes and shapes of the same virus,” Tullman-Ercek said. “Size matters for a lot of drug delivery purposes because it changes which cells are accessible and how much information can be housed inside the virus.”
Supported by the National Science Foundation and the Army Research Office, the research was reported in the journal Nano Letters. Michael Asensio and Norma Morella, graduate students in Tullman-Ercek’s laboratory, were the paper’s co-first authors.
To change the capsid’s size and shape, Tullman-Ercek used directed evolution, an engineering method that mimics the process of natural selection. Her team copied the virus’s DNA over and over to produce millions of copies, some of which had evolved and mutated. Then they looked for the mutation that made the capsid smaller.
“We weren’t expecting to see anything so drastically different from the original,” Tullman-Ercek said. “We just wanted to see what changes we could make and how much they would affect virus properties such as stability. But it turned out to be very successful.”
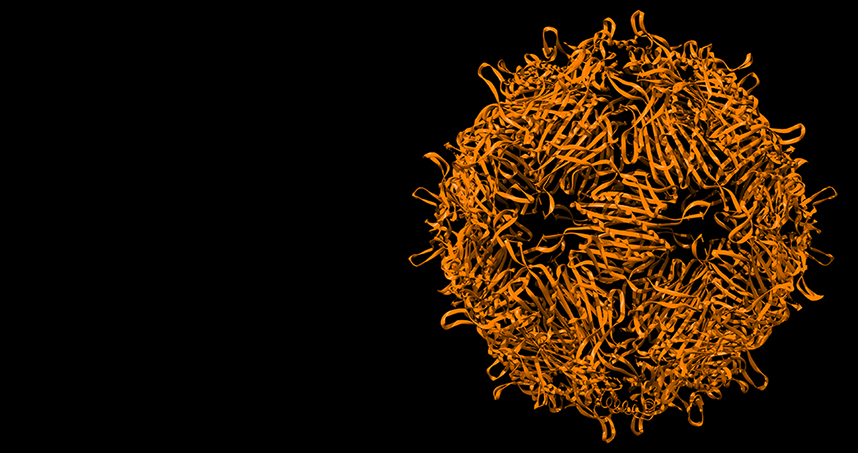
Approximately 70 percent of the generated population experienced the mutation that shrunk the capsid. While the original capsid was 180 subunits, the mutated version was has only 60 subunits. This changed the original structure form 27 nanometers to 17 nanometers. And not only was the mutated capsid smaller, it also had a different geometry.
“It changed from looking like a soccer ball with a mix of hexagons and pentagons to just having pentagons,” Tullman-Ercek said. “With just one amino acid change at the protein level, the structure completely changed and is still completely stable.”
Tullman-Ercek’s collaborator at the University of California at Berkeley plans to test the smaller virus capsid for drug delivery and compare it to the performance of the same — but larger — version of the virus. In addition to drug delivery, Tullman-Ercek said the work also raises questions about virus evolution.
“If only one amino acid change can fundamentally change the structure, then viruses can be really flexible,” Tullman-Ercek said. “Maybe viruses started small and got larger over time, and that’s why it’s so easy for them to go back to being small. We don’t know, but it’s an interesting debate.”