New Method Offers More Stable, Efficient Electrocatalytic Reactions
Approach fluidizes catalyst particles in electrolyte instead of gluing them to electrodes
Northwestern Engineering researchers have developed a more efficient and stable method to conduct electrocatalytic reactions.
The technique, which fluidizes catalyst particles in electrolyte instead of gluing them to electrodes, avoids a rapid decline in reaction performance — a phenomenon researchers call fatigue. The approach could improve production processes for electrolysis and electrochemical energy conversion and storage.
 “There has been extensive effort to find new high-performance catalysts that can also better withstand electrochemical reactions,” said Jiaxing Huang, professor of materials science and engineering at the McCormick School of Engineering, who led the research. “We developed a drastically different approach to make electrocatalysis less prone to decay — not by finding another new material, but by doing the reaction differently.”
“There has been extensive effort to find new high-performance catalysts that can also better withstand electrochemical reactions,” said Jiaxing Huang, professor of materials science and engineering at the McCormick School of Engineering, who led the research. “We developed a drastically different approach to make electrocatalysis less prone to decay — not by finding another new material, but by doing the reaction differently.”
The study, titled “Fluidized Electrocatalysis,” was published on February 10 in the journal CCS Chemistry and featured on the cover of the February issue.
In a typical electrocatalysis setting, once catalytic materials are glued onto the electrode, they are soaked in electrolyte and undergo a reaction spurred by a voltage. Since the voltage is continuously applied through the electrode, the materials experience continuous electrochemical stress. Over time, their catalytic performance can decay due to accumulated structural damage in the electrode as a whole, or on individual particles.
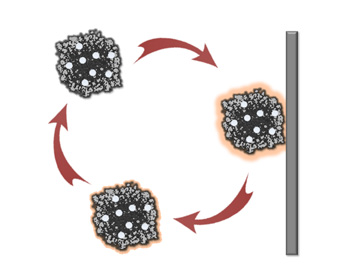
The team’s approach avoids the continuous stress by fluidizing the particles in the electrolyte. Now the particles work in rotation, experiencing electrochemical stress only momentarily when colliding with the electrode. Collectively, the output from the individual collision events merge into a continuous and stable electrochemical current.
“Fluidized electrocatalysis breaks the spatial and temporal continuum of electrochemical reactions, making the catalysts more efficient.” Huang said. “Fluidization also reduces the mass transport limit of the reactants to the catalyst, since the particles are swimming in the electrolyte.”
 Huang tested his ideas using a well-known, commercially available catalyst called Pt/C, which is made of carbon black powders decorated by platinum nanoparticles to catalyze oxygen evolution, hydrogen evolution, and methanol oxidation reactions. These three electrochemical reactions, when catalyzed by Pt/C, normally suffer from severe performance decay, but all showed higher efficiency and stability when the particles were fluidized.
Huang tested his ideas using a well-known, commercially available catalyst called Pt/C, which is made of carbon black powders decorated by platinum nanoparticles to catalyze oxygen evolution, hydrogen evolution, and methanol oxidation reactions. These three electrochemical reactions, when catalyzed by Pt/C, normally suffer from severe performance decay, but all showed higher efficiency and stability when the particles were fluidized.
“The new strategy makes an unstable catalyst deliver stable performance for all three of the model reactions. It was an exciting proof-of-concept,” said Yi-Ge Zhou, the first author of the paper and a former visiting postdoc in Huang’s group. “When we calculated single particle efficiency for some of these reactions, it was at least three orders of magnitude higher than the fixed particles. Instead of stressing them out, we gave the particles a chance to relax, and they became a lot more efficient as a result.”
While more work is needed to identify the types of electrochemical reactions that could best maximize the benefit of fluidized electrocatalysis, Huang believes his method could be applied to a variety of different types of materials and produce more efficient, longer lasting electrocatalytic reactions. This could lead to improved electrochemical synthesis processes, which play an important role in converting energy to chemicals for large-scale energy storage.
“I hope other researchers consider our method to re-evaluate their catalysts. It would be exciting to see previously deemed unusable catalysts become usable,” Huang said.
Zhou is now a professor of chemistry at Hunan University in China. Other authors include Yijin Kang, a former Northwestern visiting professor of chemistry. Northwestern’s Innovation and New Ventures Office has filed a provisional patent application based on this work.