Self-assembling Structures Open Door to New Class of Materials
Researchers at the University of Illinois and Northwestern University have demonstrated bio-inspired structures that self-assemble from simple building blocks: spheres.
The helical “supermolecules” are made of tiny colloid balls instead of atoms or molecules. Similar methods could be used to make new materials with the functionality of complex colloidal molecules. The team published their findings in the January 14 issue of the journal Science.
“We can now make a whole new class of smart materials, which opens the door to new functionality that we couldn’t imagine before,” said Steve Granick, Founder Professor of Engineering at the University of Illinois and a professor of materials science and engineering, chemistry, and physics. Granick worked with Erik Luijten, professor of materials science and engineering and of engineering sciences and applied mathematics at Northwestern’s McCormick School of Engineering, who provided the theoretical calculations and computer simulations behind the experiment.
Granick’s team developed tiny latex spheres, dubbed “Janus spheres,” that attract each other in water on one side, but repel each other on the other side. The dual nature is what gives the spheres their ability to form unusual structures, in a similar way to atoms and molecules.
In pure water, the particles disperse completely because their charged sides repel one another. However, when salt is added to the solution, the salt ions soften the repulsion so the spheres can approach sufficiently closely for their hydrophobic ends to attract. The attraction between those ends draws the spheres together into clusters.
At low salt concentrations, small clusters of only a few particles form. At higher levels, larger clusters form, eventually self-assembling into chains with an intricate helical structure.
“Just like atoms growing into molecules, these particles can grow into supracolloids,” Granick said. “Such pathways would be very conventional if we were talking about atoms and molecules reacting with each other chemically, but people haven’t realized that particles can behave in this way also.”
The team designed spheres with just the right amount of attraction between their hydrophobic halves so that they would stick to one another but still be dynamic enough to allow for motion, rearrangement, and cluster growth.
“The amount of s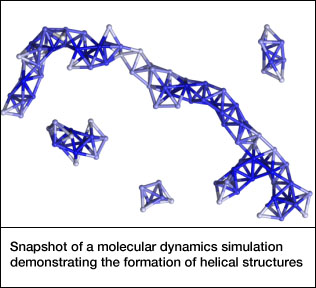 tickiness really does matter a lot. You can end up with something that’s disordered, just small clusters, or if the spheres are too sticky, you end up with a globular mess instead of these beautiful structures,” said graduate student Jonathan Whitmer, a co-author of the paper and visiting predoctoral fellow at McCormick.
tickiness really does matter a lot. You can end up with something that’s disordered, just small clusters, or if the spheres are too sticky, you end up with a globular mess instead of these beautiful structures,” said graduate student Jonathan Whitmer, a co-author of the paper and visiting predoctoral fellow at McCormick.
Surprisingly, theoretical calculations by Luijten and Whitmer showed that the most common helical structures are not the most energetically favorable. Rather, the spheres come together in a way that is the most kinetically favorable -- that is, the first good fit that they encounter.
“Through computer modeling, we confirmed that the Janus particles first aggregate into small clusters that are the basic building blocks of the helices seen in the experiments. Thermodynamics would have predicted a different type of helix, but the building block for that forms much more slowly,” Luijten said. “This shows how nonequilibrium conditions can determine what type of supracolloids you see in the lab.”
One of the advantages of the team’s supermolecules is that they are large enough to observe in real time in a microscope. The researchers were able to watch the Janus spheres come together and the clusters grow -- whether one sphere at a time or by merging with other small clusters -- and rearrange into different structural configurations the team calls isomers.
“We design these smart materials to fall into useful shapes that nature wouldn’t choose,” Granick said.
Next, the researchers hope to continue to explore the colloid properties with a view toward engineering more unnatural structures. Janus particles of differing sizes or shapes could open the door to building other supermolecules and to greater control over their formation.
“These particular particles have preferred structures, but now that we realize the general mechanism, we can apply it to other systems -- smaller particles, different interactions -- and try to engineer clusters that switch in shape,” said Granick.
The team also included University of Illinois graduate students Qian Chen and Shan Jiang and research scientist Sung Chul Bae. The U.S. Department of Energy and the National Science Foundation supported this work.