Ingredients for a New Kind of Materials Sandwich
Robert Chang and Matthew Grayson developed a superlattice that could lead to more material combinations
Superlattices are like multi-layer sandwiches with repeated layers of ingredients, and the properties they exhibit are greater than the sum of their parts, just like a triple-decker club sandwich. But those ingredients are typically restricted to crystalline materials which limits the culinary variety available to the superlattice connoisseur, like having only ham and bread in your sandwich.

Other flavors of materials include polycrystalline, made of a multitude of crystals in a single layer - think salami - and amorphous materials which have no regular structure - perhaps a thick layer of mayonnaise. But a simple brute-force layering of these materials using standard means produced unwanted defects at the interface that spoiled the flavor of the desired electronic properties.
Northwestern Engineering’s Robert Chang and Matthew Grayson have recently found the chef's secret of how to put together this new blend of ingredients.
Chang, professor emeritus of materials science and engineering at the McCormick School of Engineering, and Grayson, professor of electrical and computer engineering, realized a layered material structure first proposed in 1989 but not built until now. Superlattices have unique electronic properties because the wave-like nature of the electron allows it to sample multiple layers simultaneously, so the electronic properties transcend those of either layer separately. More candidate materials inside superlattices means a wider variety of properties and possibly brighter flat-panel displays and new classes of transistors, lasers, or solar cells.
The advancement from Grayson and his counterparts is the development of the first superlattice that is composed of different materials with different structures and physical attributes. In this work, one of the repeated layers is polycrystalline, the other layer is amorphous.
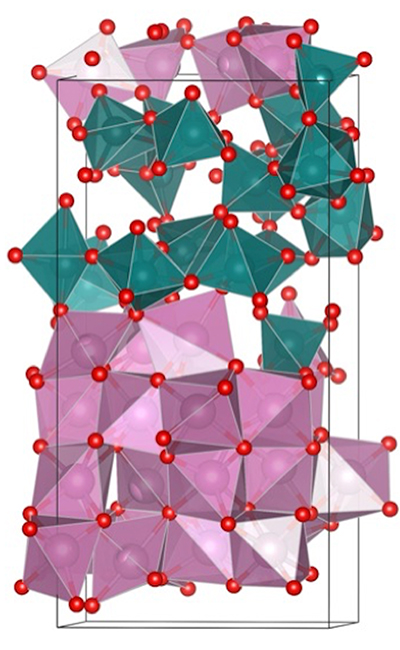
“Until now, one could only grow structures that were homomorphic, all (poly)crystalline, or all amorphous,” Grayson said. “By demonstrating such a mixed-morphology structure, it opens the door to many more materials combinations, and more materials combinations mean more composite materials properties in superlattice structures.”
Chang and Grayson presented their research in the paper “Realizing the Heteromorphic Superlattice: Repeated Heterolayers of Amorphous Insulator and Polycrystalline Semiconductor with Minimal Interface Defects,” published recently in the journal Advanced Materials.
Whereas previous attempts by researchers had failed using the materials combination of polycrystalline silicon and amorphous silicon dioxide, this work utilized polycrystalline indium oxide and amorphous molybdenum oxide, resulting in a conductive structure.
“The likely explanation is that the indium oxide tolerates increased bond angles due to its proximity to its glass transition temperature, and the result is negligible defect density at the superlattice interfaces,” Grayson said.
Building off work started a decade ago at Northwestern’s Materials Research Science and Engineering Center that included theorist Julia Medvedeva, professor of physics at the Missouri University of Science and Engineering, the team produced this structure by choosing a polycrystalline material and an amorphous material that could be deposited at the same temperature.