‘Barcoding’ DNA Vesicles for Selective Biochemical Reactions
Method increases the efficiency of DNA-mediated fusion events
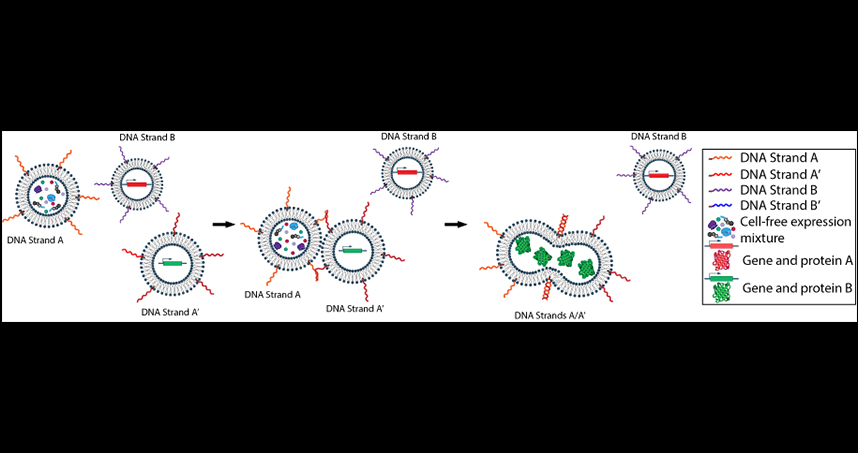
A Northwestern Engineering synthetic biology research team has developed a method to selectively drive the fusion of lipid nanoparticles — an emerging carrier for therapeutics — resulting in protein synthesis. This approach, treating DNA tethers as a molecular “Velcro,” could open the door to new and complex biochemical behaviors and transform technologies used to deliver drugs or design artificial cellular systems.
“Our method delivers molecular instructions for protein synthesis contained in one vesicle to another vesicle that contains the necessary cellular components — like ribosomes, nucleotides, and polymerases — to synthesize that protein,” said Neha Kamat, assistant professor of biomedical engineering at the McCormick School of Engineering, who led the research. “It’s the first demonstration of how to treat DNA as a sort of molecularly-specific ‘barcode’ to drive vesicles to fuse and initiate biochemical reactions.”
 The research paper, titled “Barcoding Biological Reactions with DNA-induced Vesicle Fusion,” was published on October 31 in the journal Angewandte Chemie.
The research paper, titled “Barcoding Biological Reactions with DNA-induced Vesicle Fusion,” was published on October 31 in the journal Angewandte Chemie.
Vesicles transport biological molecules to targeted cells and can be engineered to fuse to initiate reactions in cell-free systems. However, the ability to rapidly and noninvasively control the fusion between specific populations of vesicles, while limiting off-target fusion to others, has not been achieved until now.
Inspired by the ability of biological cells to send chemical messages by transporting molecules through small compartments, Kamat and her lab developed a system that optimizes two components of vesicle membranes — DNA tethers and phase-segregated membranes — to increase the efficiency of DNA-mediated fusion events. By attaching the DNA tethers to the vesicles’ exterior, the researchers could direct the fusion and delivery of DNA cargo to specific vesicle populations.
Using the DNA tethers as a molecular “Velcro” allows researchers to create any number of DNA sequences as well as benefit from the spatial and temporal freedom to selectively fuse the adjoining vesicles to spur biochemical reactions.
“Our approach uses vesicles synthesized in our laboratory and DNA molecules attached to the vesicle surface to control where and when the lipid vesicles fuse,” said Kamat, a member of Northwestern’s Center for Synthetic Biology.
DNA-mediated fusion could allow for the targeted delivery of cargo to vesicle-based bioreactors, especially when vesicles reside in hard to reach environments, like the blood stream. Targeted fusion could also allow for such bioreactors to be reloaded with new reagents, promoting longer durations of product synthesis and improving the effectiveness of therapeutic delivery.
“If you had a device implanted in your body that was designed to deliver a drug, you could use this approach to have vesicles target, fuse, deliver, and reload whatever drug it was emitting,” said Justin Peruzzi, a PhD student in Kamat’s lab and a co-first author of the study.
The approach could also help scientists conduct multiple chemical reactions while avoiding chemical crosstalk, an occurrence where all of the chemical elements mix at the same time, limiting what reactions can be generated. With the flexibility to selectively target these fusion events, multiple reactions could be executed in the same environment.
“Utilizing the compartmentalization and domains like we have allows you to encapsulate and control reactions spatially and temporally, which is an important step toward better understanding how cells work — or even creating an artificial cell,” Peruzzi said. “With the ability to selectively mix and infuse these reactions, it opens up a lot of possibilities for what we can do next.”