Electrospray Solves Longstanding Problem in Langmuir-Blodgett Assembly
The electrospray spreads water-soluble solvents on water while minimizing mixing
In the 1930s, Irving Langmuir and his colleague Katharine Blodgett were working long days in the General Electric Company’s research laboratory. Together, they discovered that by spreading molecules with volatile organic solvents on the surface of water, they could create a one-molecule-thick film and use it as an anti-reflective coating for glass. Later named Langmuir-Blodgett assembly, this thin-film fabrication technique became popular for creating molecule or nanoparticle monolayers and is commonly used until this day.
Since Langmuir-Blodgett assembly was first reported more than 80 years ago, numerous applications have been demonstrated. Yet the technique itself and the accompanying procedure have remained largely unchanged.
Now Northwestern Engineering’s Jiaxing Huang has advanced his old technique. By electrospraying materials on water’s surface, he has found a way to avoid the use of toxic organic solvents while making Langmuir-Blodgett assembly more efficient, easier to standardize, and safer to scale up.
 “The use of organic spreading solvents causes a lot of problems, especially when people use this technique to assemble thin films of nanomaterials,” said Huang, associate professor of materials science and engineering at the McCormick School of Engineering. “Nanoparticles are usually hard to disperse and can even be damaged by the solvents, leading to poor quality of the final monolayer thin films.”
“The use of organic spreading solvents causes a lot of problems, especially when people use this technique to assemble thin films of nanomaterials,” said Huang, associate professor of materials science and engineering at the McCormick School of Engineering. “Nanoparticles are usually hard to disperse and can even be damaged by the solvents, leading to poor quality of the final monolayer thin films.”
Now available online, the paper describing Huang’s research was published in the Journal of the American Chemical Society (JACS).
Compared to other thin-film fabrication techniques, Langmuir-Blodgett assembly has unique advantages, including its fine control over the packing density in the monolayer, which can stack up to form films with precise number of layers. This only works, however, if the nanoparticles are well dispersed in both the spreading solvents and on the water’s surface.
“This is quite challenging,” Huang said. “It requires a very delicate balance of surface properties for nanoparticles to be well dispersed on water’s surface and in those water-hating solvents.”
“There are also other problems,” said Hua-Li Nie, a former visiting scholar in Huang’s lab from Donghua University and the paper’s first author. “Nanoparticles made of polymers, organic compounds, or biological materials tend to be damaged in organic spreading solvents.”
“These volatile organic solvents also pose a safety hazard,” Huang added. “It becomes problematic when we think about manufacturing.”
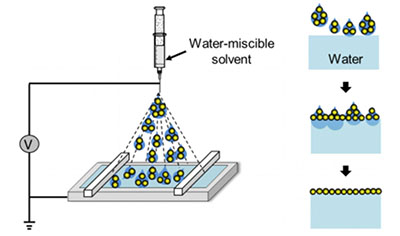 Using a water-soluble, benign solvent, such as ethanol, to spread the nanoparticles avoids all of these problems except one: the solvent mixes with water, causing the particles to sink.
Using a water-soluble, benign solvent, such as ethanol, to spread the nanoparticles avoids all of these problems except one: the solvent mixes with water, causing the particles to sink.
“People have come up with many tricks to deal with this problem of sinking particles,” Huang said. “One way is to very gently disperse ethanol on water’s surface to avoid excessive mixing, but there is still a lot of wasted material.”
Huang’s group even used this strategy to demonstrate Langmuir-Blodgett assembly of graphene oxide sheets, which was featured on the cover of JACS in 2009.
Huang discovered that he could prevent the solvent from mixing with the water by applying it with an aerosol spray. The spray breaks the solvent, which contains the nanoparticles, into millions of smaller droplets.
“If you reduce the size of the droplets to the micron range, they won’t ‘bombard’ the water’s surface,” Huang said. “Think about a humidifier: the water droplets float around because they are not very sensitive to gravity.”
More importantly, because of their very small volume, by the time the droplets spread on the water, they are consumed immediately. “Mixing with water is suppressed because there’s nothing left to mix,” Huang said.
With electrospray, one does not have to rely on “skillful hands” to perform good Langmuir-Blodgett assembly. Huang said electrosprays can be automated to standardize and scale up this technique for manufacturing. Not only would this save time and money, it is also much safer. Many of the popularly used organic spreading solvents, such as chloroform, are toxic. This requires the water trough to be kept in a controlled environment, which can be costly to construct and maintain as well as dangerous. Now researchers can choose any type of benign liquid as a solvent.
“We’ve broadened the landscape for Langmuir-Blodgett assembly,” Huang said. “A very simple idea — reducing the droplet’s size — has solved a longstanding problem for an old but popular technique.”