New COVID-19 Nasal Spray Outperforms Current Antibody Treatments in Mice
A single inhaled dose treated or even prevented infection by COVID-19 and its variants
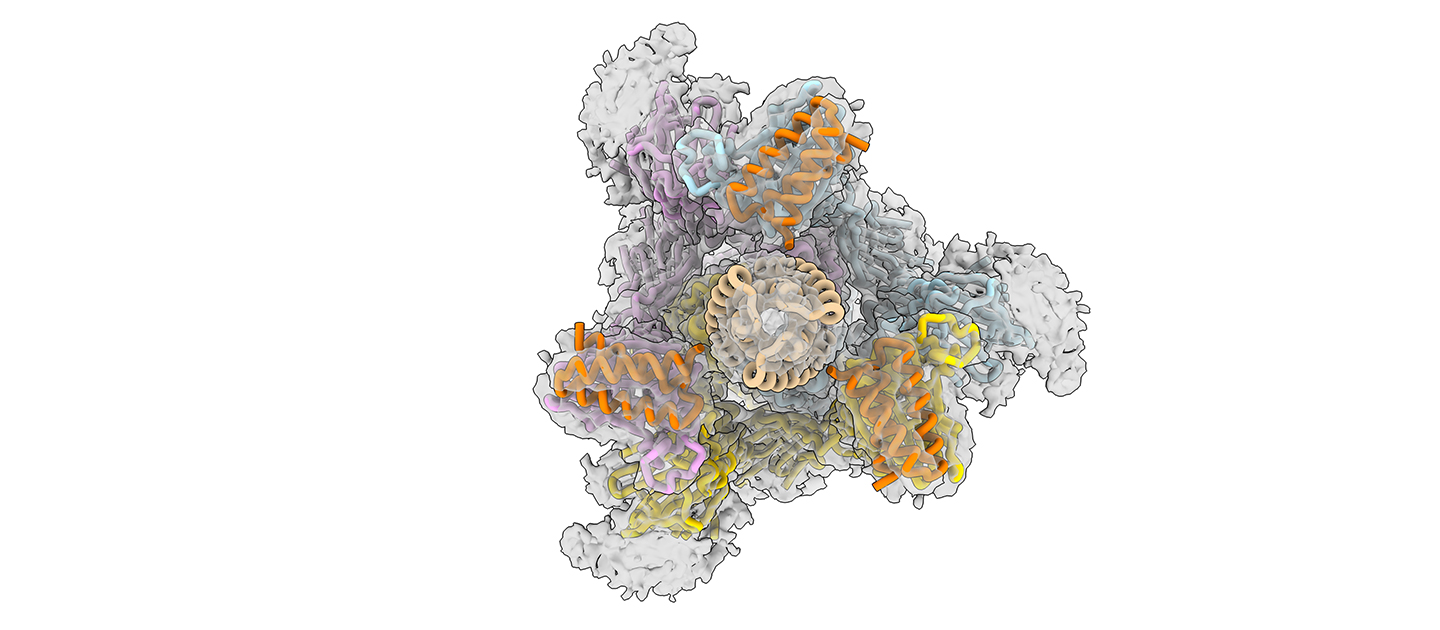
A new protein-based antiviral nasal spray developed by researchers at Northwestern University, the University of Washington, and Washington University at St. Louis is being advanced toward Phase I human clinical trials to treat COVID-19.
Designed computationally and refined in the laboratory, the new protein therapies thwarted infection by interfering with the virus’ ability to enter cells. The top protein neutralized the virus with similar or greater potency than antibody treatments with Emergency Use Authorization status from the US Food and Drug Administration (FDA). Notably, the top protein also neutralized all tested SARS-CoV-2 variants, something that many clinical antibodies have failed to do.
When researchers administered the treatment to mice as a nasal spray, they found that the best of these antiviral proteins reduced symptoms of infection — or even prevented infection outright.

The findings were published April 12 in the journal Science Translational Medicine.
This work was led by Northwestern Engineering’s Michael Jewett; David Baker and David Veesler at the University of Washington School of Medicine; and Michael S. Diamond at WashU.
To begin, the team first used supercomputers to design proteins that could stick to vulnerable sites on the surface of the novel coronavirus, targeting the spike protein. This work was originally reported in 2020 in the journal Science.
In the new work, the team reengineered the proteins — called minibinders — to make them even more potent. Rather than targeting just one site of the virus’ infectious machinery, the minibinders simultaneously bind to three sites, making the drug less likely to detach.
“SARS-CoV-2’s spike protein has three binding domains, and common antibody therapies may only block one,” Jewett said. “Our minibinders sit on top of the spike protein like a tripod and block all three. The interaction between the spike protein and our antiviral is among the tightest interactions known in biology. When we put the spike protein and our antiviral therapeutic in a test tube together for a week, they stayed connected and never fell apart.”
Jewett is a professor of chemical and biological engineering at Northwestern’s McCormick School of Engineering and director of Northwestern’s Center for Synthetic Biology. Andrew C. Hunt, a graduate research fellow in Jewett’s laboratory, is the paper’s co-first author.
As the SARS-CoV-2 virus has mutated to create new variants, some treatments have become less effective in fighting the ever-evolving virus. Just last month, the FDA paused several monoclonal antibody treatments, for example, due to their failure against the BA.2 omicron subvariant.
To enter the body, the spike protein and ACE2 receptor engage in a handshake. Our antiviral blocks this handshake and, as a bonus, has resistance to viral escape. Walter P. Murphy Professor of Chemical and Biological Engineering
Unlike these antibody treatments, which failed to neutralize omicron, the new minibinders maintained potency against the omicron variant of concern. By blocking the virus’ spike protein, the new antiviral prevents it from binding to the human angiotensin-converting enzyme 2 (ACE2) receptor, which is the entry point for infecting the body. Because the novel coronavirus and its mutant variants cannot infect the body without binding to the ACE2 receptor, the antiviral also should work against future variants.
“To enter the body, the spike protein and ACE2 receptor engage in a handshake,” Jewett said. “Our antiviral blocks this handshake and, as a bonus, has resistance to viral escape.”
In addition to losing effectiveness, current antibody therapies also come with several problems. They are difficult to develop, expensive, and require a healthcare professional to administer. They also require complicated supply chains and extreme refrigeration, which is often unavailable in low-resource settings.
The new antiviral solves all these problems. As opposed to monoclonal antibodies, which are made by cloning and culturing living mammalian cells, the new antiviral treatment is produced large-scale in microorganisms like E. coli, making them more cost-effective to manufacture. Not only is the new therapy stable in high heat, which could further streamline manufacturing and decrease the cost of goods for clinical development, it also holds promise for being self-administered as a one-time nasal spray, bypassing the need for medical professionals.
The researchers imagine that it could be available at the pharmacy and used as a preventative measure to treat infections.