Engineering Therapeutics
Taking aim at disease
Diseases like cancer and HIV continue to kill millions of people each year. Mental illnesses like depression and schizophrenia continue to shatter lives. Although therapies for such diseases have improved dramatically in recent years, they often fall short of ideal. In many cases, problems with efficacy lie not in the treatments, but in the targets.
For example, chemotherapy can effectively arrest cancer, but it attacks healthy cells as well as tumors, and still only cures the disease part of the time. Even when life is prolonged, the side effects of enduring a chemical onslaught can prove painful and debilitating. If such therapies could be contained and honed to target only mutated cells while sparing the healthy ones, treatment would be more bearable and, most likely, more successful.
Enabled by advances in nanotechnology, molecular therapeutics, genomics, and computation, as well as a better understanding of disease mechanisms, researchers at the McCormick School of Engineering are using design thinking to explore creative new ways to treat such diseases. Instead of developing new therapeutics, they’re focused on finding better methods and new targets for delivering existing drug therapies.
Patrick Kiser
New Delivery Target: The Reproductive System

Using tenofovir is not a new idea: three-and-a-half million HIV- infected people worldwide take an oral version of it. Kiser’s break- through is a device that reaches a new target—the female reproductive system. Using a previously unexplored methodology, the TDF intravaginal ring delivers tenofovir directly to the site where transmission takes place, requiring a much lower dose than oral pills and eliminating potentially serious side effects, including reduced kidney function.
Within one year, Kiser invented the TDF intravaginal ring, tested it in non-human primates, where it demonstrated a 100 percent success rate, and then obtained approval from the FDA for clinical testing. The ring is currently undergoing its first test in women in a Phase I clinical trial in New York.
“This and other long-acting delivery systems could be an important tool in preventing rampant HIV transmission in many low-income countries,” say Kiser, who is an associate professor of biomedical engineering.
Expanding the scope
Closer to Kiser’s home in Chicago, the HIV pandemic looks much different than in developing countries. In Chicago, African-American gay men are the population at highest risk for HIV.

Exploring new applications
To reach a wider segment of the population, Kiser is working on long-acting implants that could be used by anyone susceptible to HIV. The implant is a small rod, no thicker than a matchstick, that when inserted beneath the skin in the arm could deliver antiretroviral drugs consistently for many months. The rod implant could be made from similar technology as the ring: an antiretro- viral held in a medical-grade plastic shell that controls the drug’s diffusion, so it is released in a controlled manner from the device and into the circulation.
Kiser continues to find new uses for his intravaginal ring innovation. His lab was the first to invent a ring that can prevent HIV, herpes, and unwanted pregnancy by delivering tenofovir and the hormone levonorgestrel in combination. This ring, also soon to enter clinical trials, has reliably delivered both drugs for three months. Also under development by a company he started is Kiser’s “Aquaring,” which dispenses lubricant for women with vaginal dryness.
“What my lab brings to the table is the ability to do creative drug delivery work and help see that through the clinic,” he says. “We’ve had five rings in the past two years tested in humans. That’s a major feat for an academic lab.”
Igal Szleifer
New Delivery Method: Liposomes

Igal Szleifer has been interested in modeling new systems for drug delivery for his entire 25-year career. He says his job is “to think about all the possibilities.”
“We don’t come up with the ultimate solution using theory and modeling,” says Szleifer, the Christina Enroth-Cugell Chair of Biomedical Engineering at McCormick. “We narrow the variables in order to get an experiment to work, so others can concentrate on things that are doable in a relatively short time frame rather than having to test all the possibilities.”
For many years, Szleifer has studied spherical vesicles composed of lipids, called liposomes, a group of naturally occurring molecules that includes fats and waxes. Tiny, bubble-like structures, liposomes are made from the same materials as cell membranes and can be filled with therapeutics, such as drugs or DNA.
“Liposomes make great drug delivery systems because they can be 100-percent biodegradable and biocompatible,” he says. “Because the membrane is two molecules thick, the loading of the drug is very high and release can be triggered by disrupting subtle interactions in the bilayer. This is a beautiful example of how manipulating nanostructures can lead to important applications.”
Recently, Szleifer’s team has modified the surfaces of liposomes with pH-sensitive peptides. When the liposome enters a more acidic environment, the peptide senses the change and delivers the drug. This quality could prove particularly useful for cancer treatment.
“The environment of cancerous tumors is more acidic than normal tissue,” Szleifer says. “We can tailor features of drug delivery systems to take advantage of that.” Szleifer’s team is also modeling how different concentrations of cholesterol in the liposome can optimize drug delivery. Cholesterol makes the liposome’s membrane tighter, slowing diffusion of the drug. The team discovered that cholesterol also affects the placement of the drug in the liposome; it changes the position of where the drug spends time before exiting the membrane. Knowing the drug’s position helps researchers better understand its release profile.
Collaborating on targeted therapeutic delivery
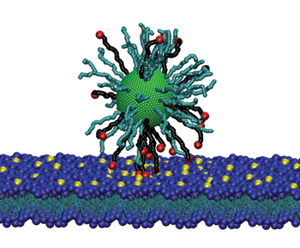
Szleifer is also creating models to help his fellow McCormick researcher, Patrick Kiser, understand the diffusion of drugs from Kiser’s innovative intravaginal rings. For example, to be completely effective as a contraceptive, Kiser’s tenofovir levonorgestrel combination ring should be left in place for the entire three months that it’s active.
In clinical trials, however, researchers found that some women removed the ring for short periods of time for various reasons. How effective is it if the ring is removed and the drug concentration drops? For the answer, Kiser turned to Szleifer to build a theoretical model of the female reproductive system to understand the distribution and concentrations of the drugs in vaginal tissues.
Another member of the team, Thomas Hope, professor of cell and molecular biology at the Feinberg School of Medicine and a leading HIV virologist/immunologist, wants to know where the HIV goes in the body and how that overlaps with the drug as it disperses. Szleifer’s model will help determine the best dosages and delivery format to intercept the virus.
“We want to not only discover what dose is needed to prevent infection by the virus, but we also want to know what the levels are in the cells that get infected and the probability of interaction with the virus,” Szleifer says. “To stop this virus we need to quantitatively understand how the drug and target cells interact with the virus during the first steps of infection.” A combined approach using engineering, virology, and modeling is required for the next breakthrough.
Samuel Stupp
New Delivery Method: Cargo Compartments for Drugs

Fifteen years ago, when Samuel Stupp first developed a cylindrical nanofilament structure that mimicked the collagen fibers present in bone materials, he knew the nanofilaments had great potential for applications in regenerative medicine.
Although Stupp, the Board of Trustees Professor of Materials Science and Engineering, Chemistry, Medicine, and Biomedical Engineering, never expected to apply this work to the area of drug delivery, he recently discovered that the nanofilaments showed promise in that area as well.
“You can’t always predict what’s going to happen,” says Stupp. “During experiments, we realized these filaments had a great capacity to interact with cells, and they are biocompatible. Using them to deliver drugs was a natural idea.”
Finding the right “zip code”
On further investigation, Stupp discovered that the nanofilaments could encapsulate drugs in their hydrophobic cores, and slowly release the drugs as they biodegrade. “Think of the core of the cylinder as a cargo compartment,” he says.
"During experiments, we realized these filaments had a great capacity to interact with cells, and they are biocompatible. Using them to deliver drugs was a natural idea."
Using the cylindrical nanofilaments to encapsulate chemotherapy drugs, Stupp’s team found they were effective in reducing tumor size in an animal model of breast cancer, work that has been supported by Northwestern’s Center of Cancer Nanotechnology Excellence. Stupp’s team also incorporates targeting information on the surface of the cylinder, guiding it to a specific location in the body.
“It’s almost like you provide information that allows the cylinder to find a ZIP code,” he says. That “ZIP code” can take many forms; one of them is a structure that binds to common receptors in cancer cells. Another possibility is to rely on the fact that tumors express certain types of enzymes at a level that normal tissues do not. Thus, the ZIP code could be the substrate for those enzymes, and when the cylinders encounter a cancerous environment, they are more likely to break apart and release the drug they’re carrying.
Uncovering new applications
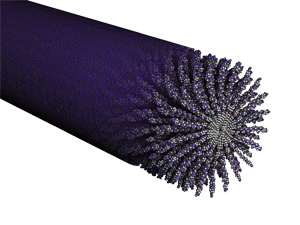
Stupp says the nanofilaments can also perform double duty— carrying drugs not only inside their compartments, but also bonded to their surfaces. Because it’s difficult to know how much of the drug a cylinder can hold in its core, bonding the drug to the surface increases its capacity so it can deliver a larger dosage.
“Then we could have the possibility of having a nanostructure that releases two drugs,” Stupp adds. “Maybe the drugs need to be released at different rates, so one is held inside and one outside.”
Most recently, Stupp’s team has used the cylinders to store and deliver therapeutic gases, which have important biological effects but are typically unstable and difficult to corral and control. He has experimented with delivery of nitric oxide in collaboration with vascular surgeon Melina Kibbe in the Feinberg School of Medicine. This naturally produced gas can prevent the closing of blood vessels after stent placement.
“Stent placement, a very common procedure, saves many lives each year,” Stupp says. “We put targeting information—the ZIP code—on the outside of the nanofilament, and that ZIP code delivered the cylinder to the place where the artery had been injured, and then it released the gas. It showed great success.”
Monica Olvera De La Cruz
New Delivery Method: Nanocontainers

For inspiration, Monica Olvera de la Cruz looks to the depths of the Dead Sea, where bacterial halophiles thrive under extreme conditions. From the Greek word for “salt-loving,” halophiles live in water with exceptionally high salt concentrations, an environment where very few organisms can survive.
“The organisms are protected because their wall membranes are crystalline,” says Olvera de la Cruz, the Lawyer Taylor Professor of Materials Science and Engineering. “This creates a very strong surface. Otherwise, the salt would kill them.”
Using this principle drawn from nature, Olvera de la Cruz’s team has designed liposome “nanocontainers,” knowing that when liposomes crystallize, they become more stable and can withstand transportation into and through the human body.
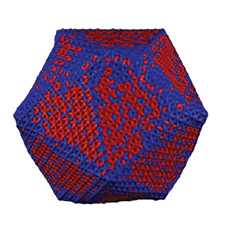
To achieve this desired result, Olvera de la Cruz uses the electrostatics of chemical compounds that have heads and tails, called lipid amphiphiles. The heads are attracted to water; the tails are repelled by it. Her team computationally combined three amphiphiles with positive charges and one with a negative charge, which turned the liposomes into different shapes. They became multifaceted polyhedrons, with flat faces, straight edges, and sharp corners.
When broken symmetries occur, the liposomes have a “directional” reaction to stimuli, resulting in specific changes that induce other processes, such as the attraction to a preferred location in the body and release of the drug. “The less symmetry there is, the more function you have,” says Olvera de la Cruz. “If everything were homogenous, then nothing would happen in nature.”
Since changing the shape of liposomes at the nanolevel also changes their mechanics, researchers can design liposomes with specific shapes that can be recognized by receptors in the body to perform different functions. The surfaces can be patterned with components that are drawn toward a specific target, like a tumor, where the drug is released. The crystallized shell of the liposome not only helps the drug find its target, but also acts as a strong barrier that slowly breaks down for a timed release. This slow release is ideal because the therapeutic is delivered in a large, single dose that diffuses over time rather than in smaller doses that must be administered time and again.
Joseph Moskal
New Delivery Target: Brain Receptors for Learning and Memory

People suffering from treatment-resistant depression can go months or even years without any relief. Finally, for them, there may be a light at the end of the tunnel. A new antidepressant drug created by Joseph Moskal has been tested on adults who have not been helped by other antidepressant therapies, and it alleviated symptoms within hours.
“This addresses a population in great need of something that works,” says Moskal, research professor of biomedical engineering at McCormick and founder of neuropharmaceutical company Naurex.
Looking from a different angle
The road to Moskal’s discovery began when he was a senior staff fellow at the National Institutes of Health, where he studied monoclonal antibodies to understand pathways of learning and memory. He realized this area of the brain could have potential for the treatment of neurological disease.
To create relief for difficult-to-treat depression, Moskal looked at the condition from a different angle. Instead of targeting serotonin receptors, as many antidepressants do, Moskal targeted brain receptors responsible for learning and memory—a very different approach from existing antidepressants.
"The drug has potential to address major issues that confront millions of patients worldwide."
The resulting drug, GLYX-13, is a four amino acid peptide that modulates NMDA (N-Methyl-D-aspartate) receptors in the brain. NMDA receptors play a key role in regulating the quality of the connection between neurons—synaptic plasticity—and are important in regulating learning and memory functions.
In clinical trials administered at 12 sites nationwide, a single dose of GLYX-13 resulted in significant reductions in depression symptoms among subjects who had shown little improvement with previous drugs. The positive effects of GLYX-13 were evident within 24 hours and lasted an average of seven days. The effect size, a measure of the magnitude of the drug’s antidepressant effcacy, at both these times after a single dose was nearly double the effect size seen with most other antidepressant drugs after four to six weeks of repeated dosing. In addition, GLYX-13 does not share the serious side effects, such as hallucinations and schizophrenia-like symptoms, of another drug on the market, ketamine, which also targets NMDA receptors.
While GLYX-13 is administered intravenously, Moskal is also working on an oral drug with similar properties and potential. The compound recently completed Phase IIb clinical trials and received a fast track designation from the Food and Drug Administration to expedite development.
The new drug also could be helpful in treating other neurological conditions, including schizophrenia, bipolar disorder, anxiety, and Alzheimer’s disease. Moskal says, “The drug has potential to address major issues that confront millions of patients worldwide.”
