Cells’ Collective Behavior Determines Tissue Shapes
Collective modes of cellular deformations influence the physical shapes of developing tissues
A team of researchers, including Northwestern Engineering’s Madhav Mani, has brought the world closer to understanding how living tissues and cells arrive at their physical forms.
By studying a developing fruit fly embryo, the team has identified a collective mode that provides a quantitative description of the deforming epithelial tissue. Such deformation, coupled with the mechanics of growth, then defines the shape of the developing tissues.
 “While the community of developmental biologists has an ever-growing molecular parts list, we are still at a loss as to how development proceeds,” said Mani, assistant professor of engineering sciences and applied mathematics in Northwestern’s McCormick School of Engineering. “We are trying to quantitatively describe how morphogenesis — or the emergence of physical form — occurs.”
“While the community of developmental biologists has an ever-growing molecular parts list, we are still at a loss as to how development proceeds,” said Mani, assistant professor of engineering sciences and applied mathematics in Northwestern’s McCormick School of Engineering. “We are trying to quantitatively describe how morphogenesis — or the emergence of physical form — occurs.”
Supported by the National Science Foundation and Gordon and Betty Moore Foundation, the research was published last week in Nature Physics. Boris I. Shraiman, the Susan F. Gurley Professor of Theoretical Physics and Biology at the University of California at Santa Barbara, was the paper’s corresponding author. Nicholas Noll, a graduate student in Shraiman’s laboratory, served as the paper’s first author.
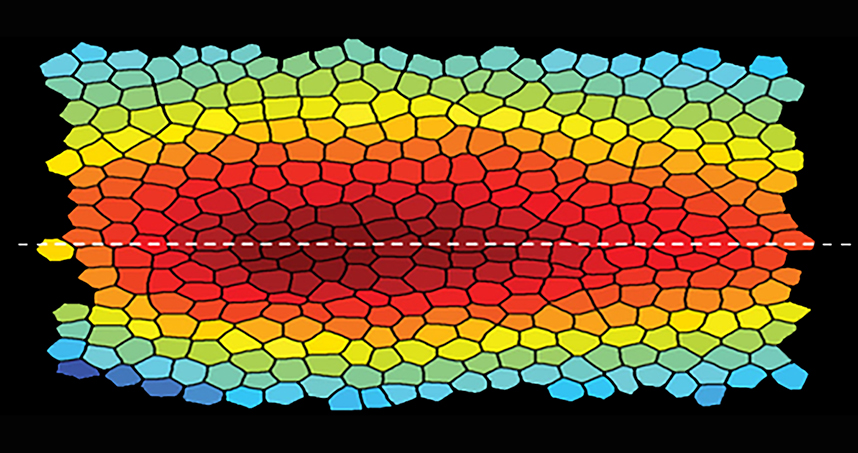
Epithelial tissue is a sheet of cells that makes up the outer-most layer of skin, lines body cavities, and covers organ walls. Fitting closely together to form sheets, epithelial cells are differently sized and shaped and can be arranged into differently shaped structures. Each cell is linked to its neighboring cells, resulting in a mechanical network that ensures the tissue’s structural integrity.
To better understand the mechanical interactions at play in epithelial morphogenesis, the team formulated and analyzed a model of epithelial tissue as a two-dimensional active tension network (ATN), which assumes that the outer layer of tissue determines the mechanical balance within its cells. The model showed a dynamic relationship between cells and their neighbors.
“If you watch people at a busy crosswalk, you will notice that individuals don’t appear to walk independently,” Mani said. “They constantly watch each other, move in response to others’ movements, and thereby move as a whole with certain collective traits. Cells behave similarly when entire tissues flow in an embryo. Our paper uses quantitative analysis and physical modeling to understand the collective dynamics, shedding light on the mechanisms that might be at play at the molecular scale.”
Mani and his team discovered that epithelial tissues in the ATN model exhibited unusual behaviors. Juxtaposing inanimate matter, the tissue exhibits fluid-like behavior at short time scales and solid-like behavior at longer time scales. The researchers believe this “active solid” behavior arises from the tissues’ dynamic recruitment of myosin, a type of motor protein that plays a role in muscle contraction.
Mani said this information gives the field one of the first “stamps” for its collection. To further understand cells’ collective behaviors, his team will pursue a combination of live-imaging experiments, mathematical modeling, and quantitative analysis.
“The hope is that more general principles will emerge once we have a few more ‘stamps’ from different settings,” Mani said.